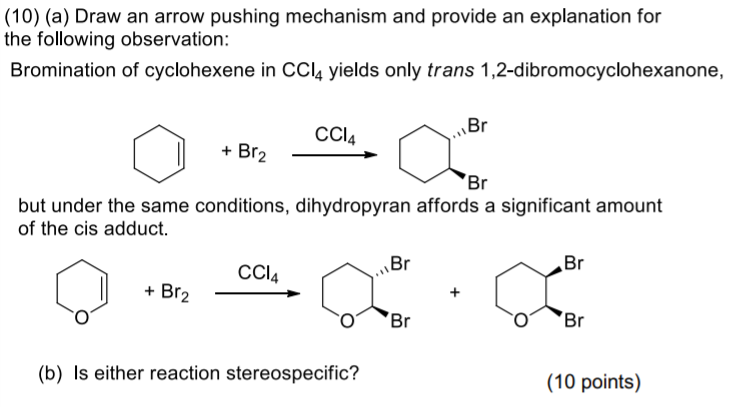
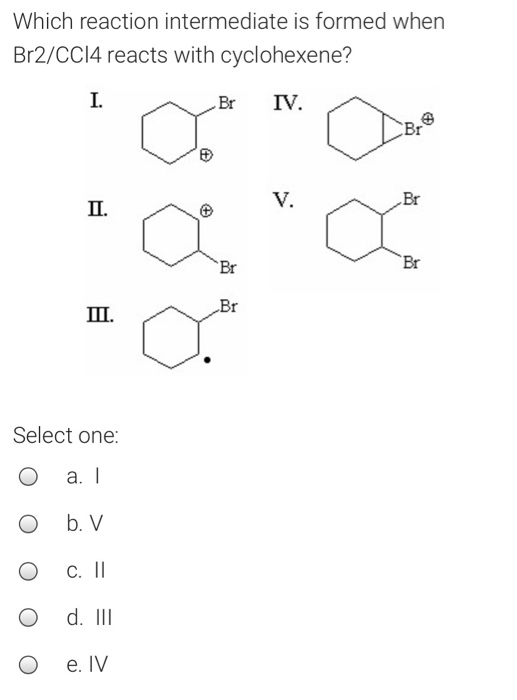
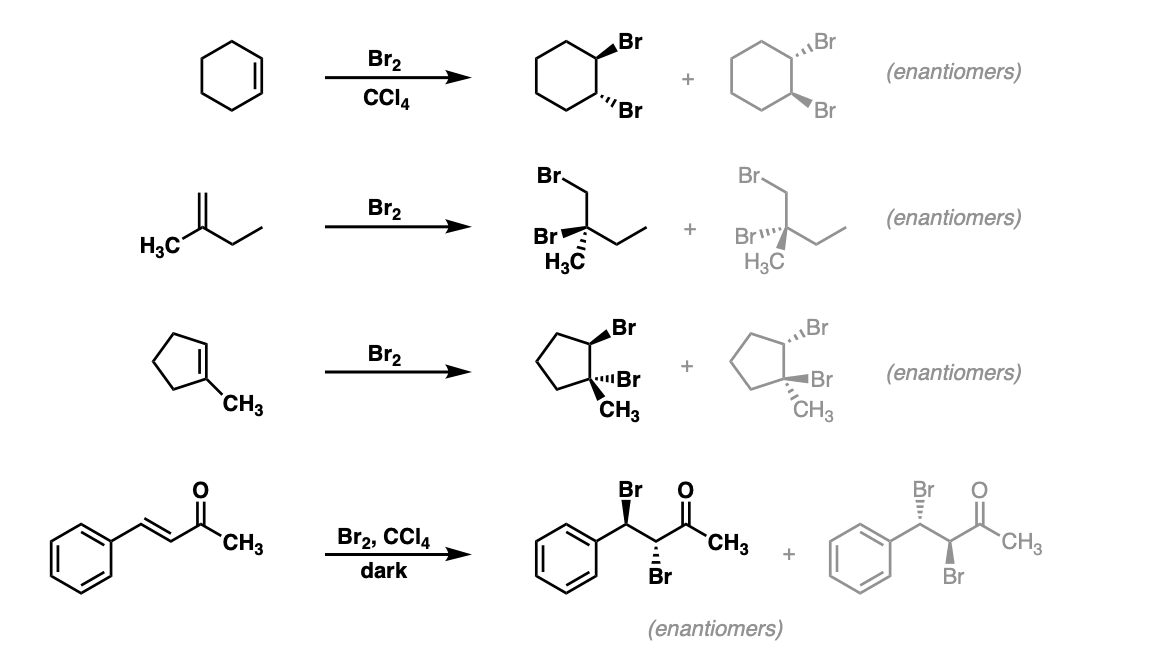
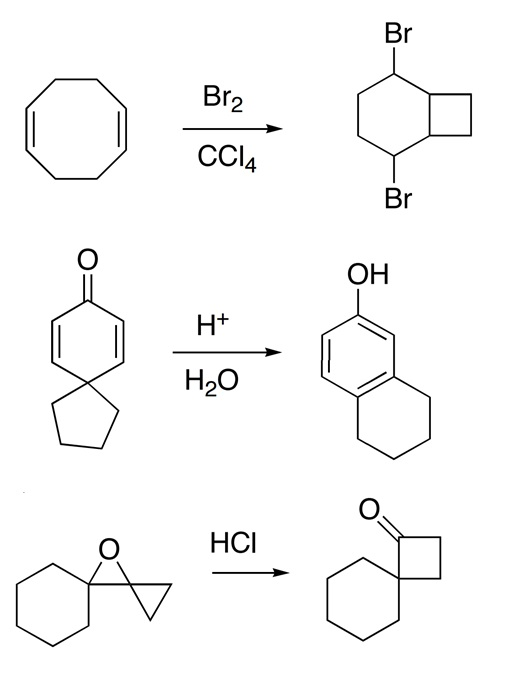
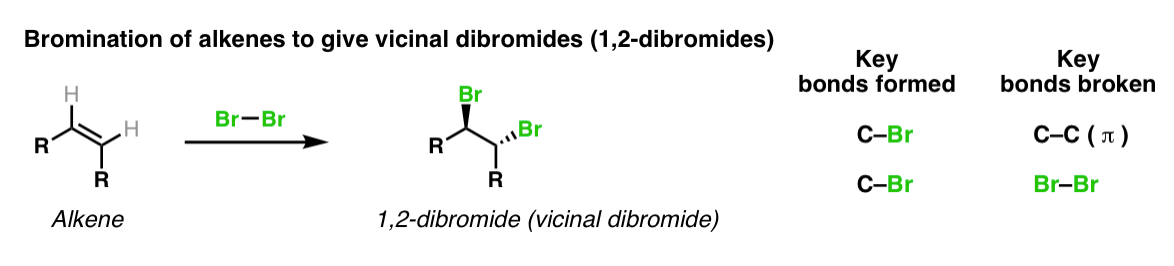
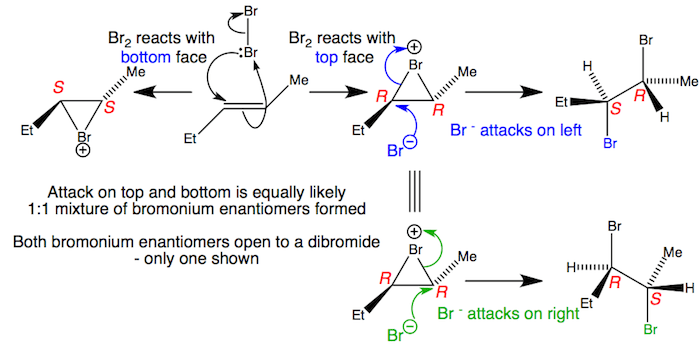
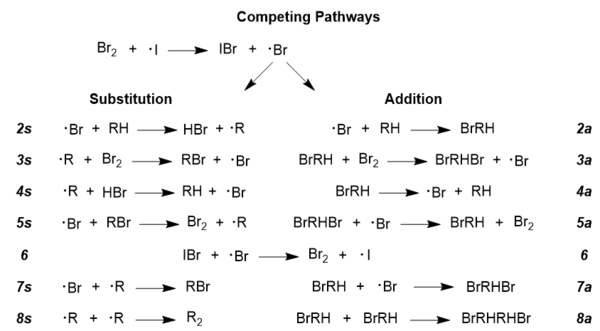
organic chemistry - Bromination of 1,3,5-cyclooctatriene followed by nucleophilic substitution by excess dimethylamine - Chemistry Stack Exchange
Cyclohexene is reacted with bromine in CCl4 in the dark. The product of the reaction is- - Sarthaks eConnect | Largest Online Education Community

aromaticity_SB (1) - 3. Reactions of Benzene Br2 CCl4 Br2 CCl4 Br Br No Reaction Ch. 14 - 1 1. OsO4 2. NaHSO3 1. OsO4 2. NaHSO3 OH OH No Reaction Ch. 14 | Course Hero
Cyclohexene is reacted with bromine in CCl4 in the dark. The product of the reaction is- - Sarthaks eConnect | Largest Online Education Community

![CH3 - CH3 |CH - CH2 - CH3 + Br2 []hv P (Major product) (on monobromination) The product P is : CH3 - CH3 |CH - CH2 - CH3 + Br2 []hv P (Major product) (on monobromination) The product P is :](https://d2rrqu68q7r435.cloudfront.net/images/2384759/c98e3b98-460c-49b2-84d5-01a8bfbf2995.jpg)