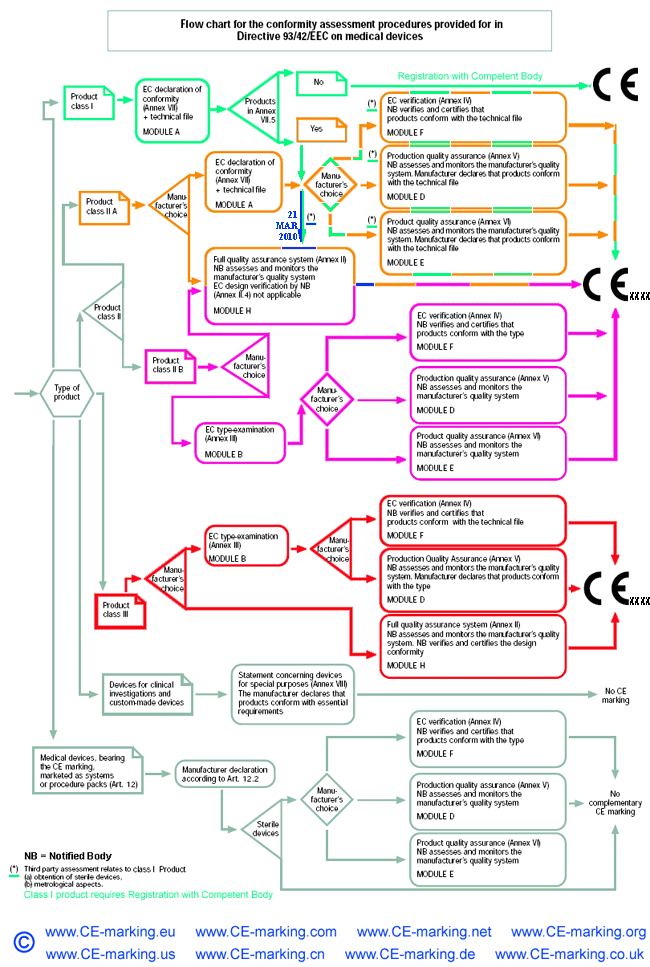
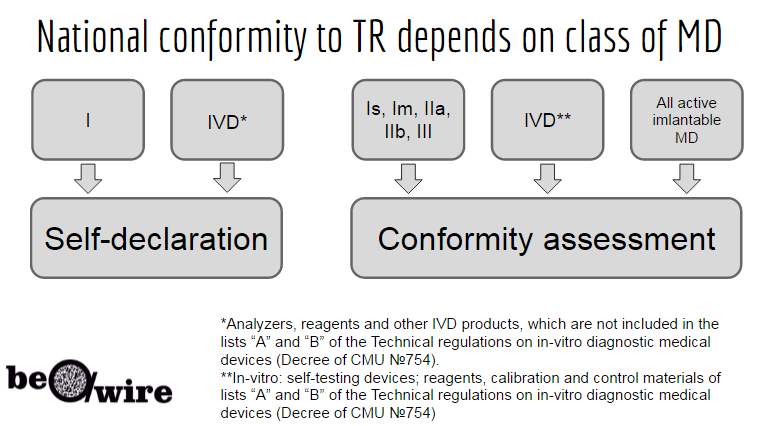
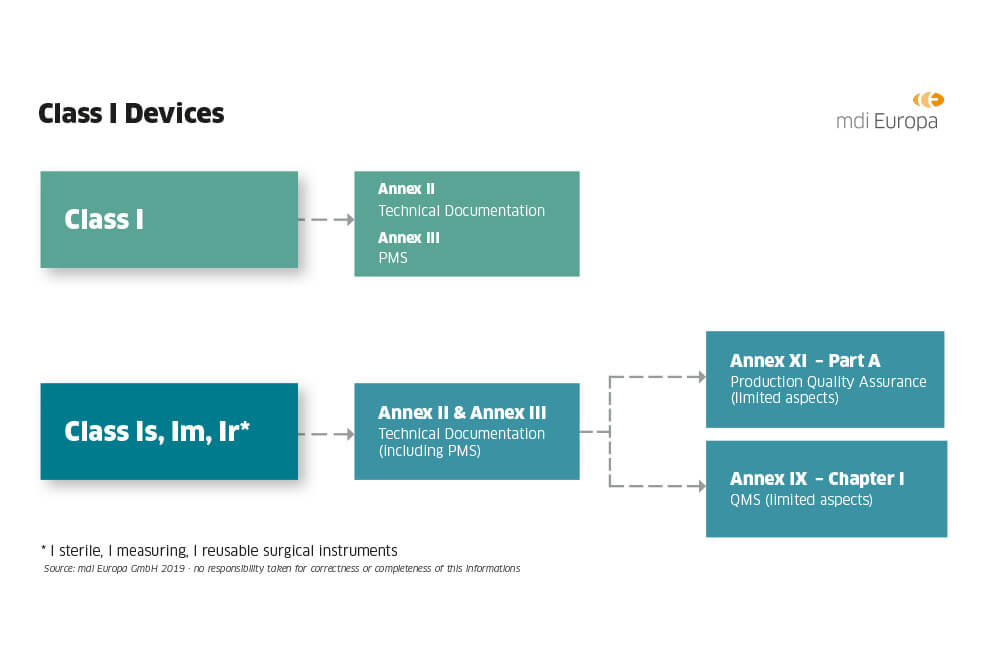
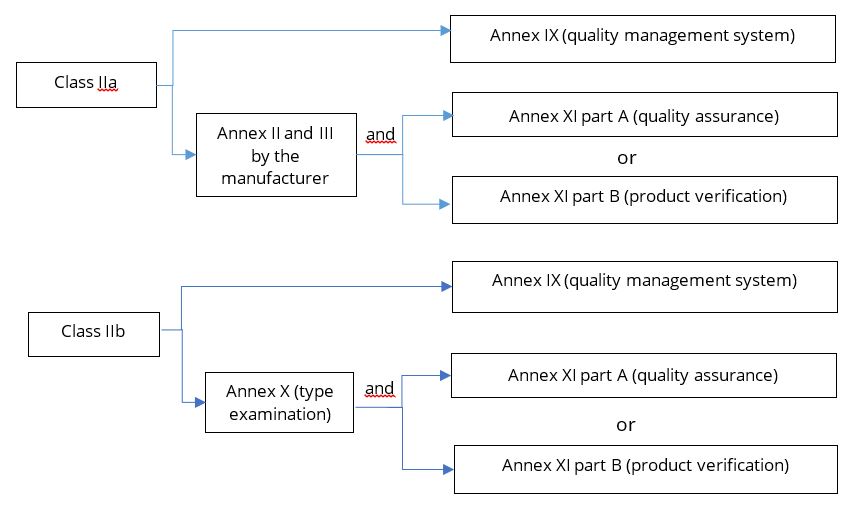
DEVICE REGULATIONS - The New Medical Device Regulation & the Applicability of Article 117 to Medicinal Products

Medical Devices. Notified Bodies and the CE certification Process for Medical Devices. European Surgical Robotics Demonstration Day - PDF Free Download
Availability and capacity of notified bodies to carry out conformity assessments for COVID-19 related medical devices and in vit