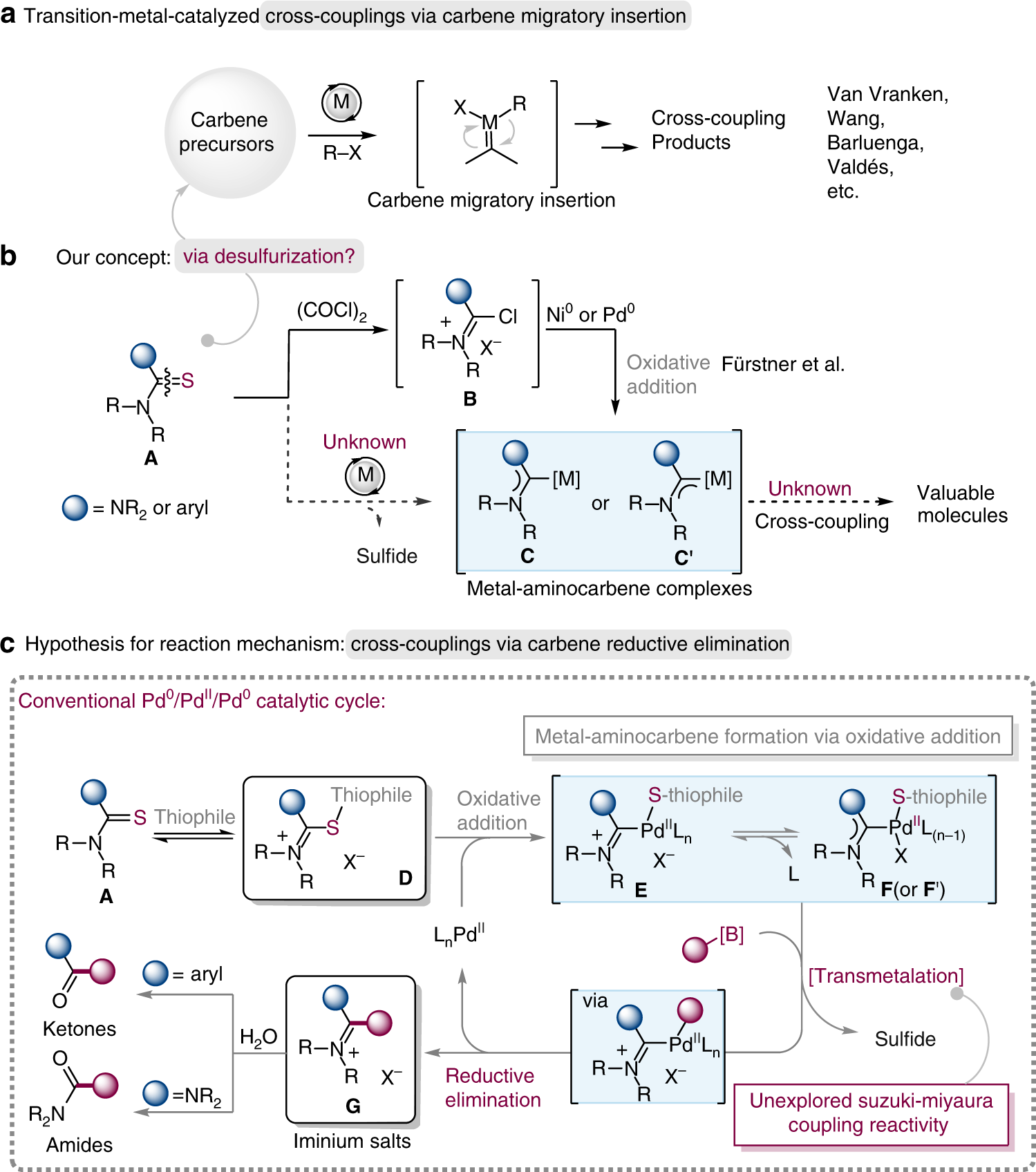
Catalysts | Free Full-Text | Palladium-Catalyzed Suzuki–Miyaura Cross- Coupling in Continuous Flow | HTML
Selection of boron reagents for Suzuki–Miyaura coupling - Chemical Society Reviews (RSC Publishing) DOI:10.1039/C3CS60197H

organic chemistry - Why does thallium hydroxide increase the yield of product in a Suzuki reaction? - Chemistry Stack Exchange

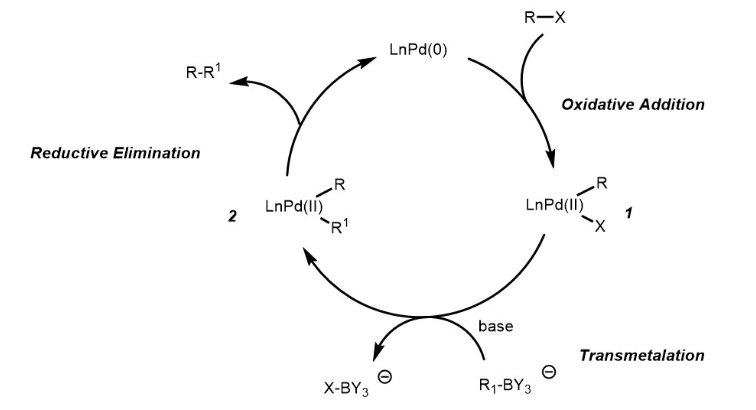
Mechanistic Aspects of the Palladium‐Catalyzed Suzuki‐Miyaura Cross‐Coupling Reaction - D'Alterio - 2021 - Chemistry – A European Journal - Wiley Online Library

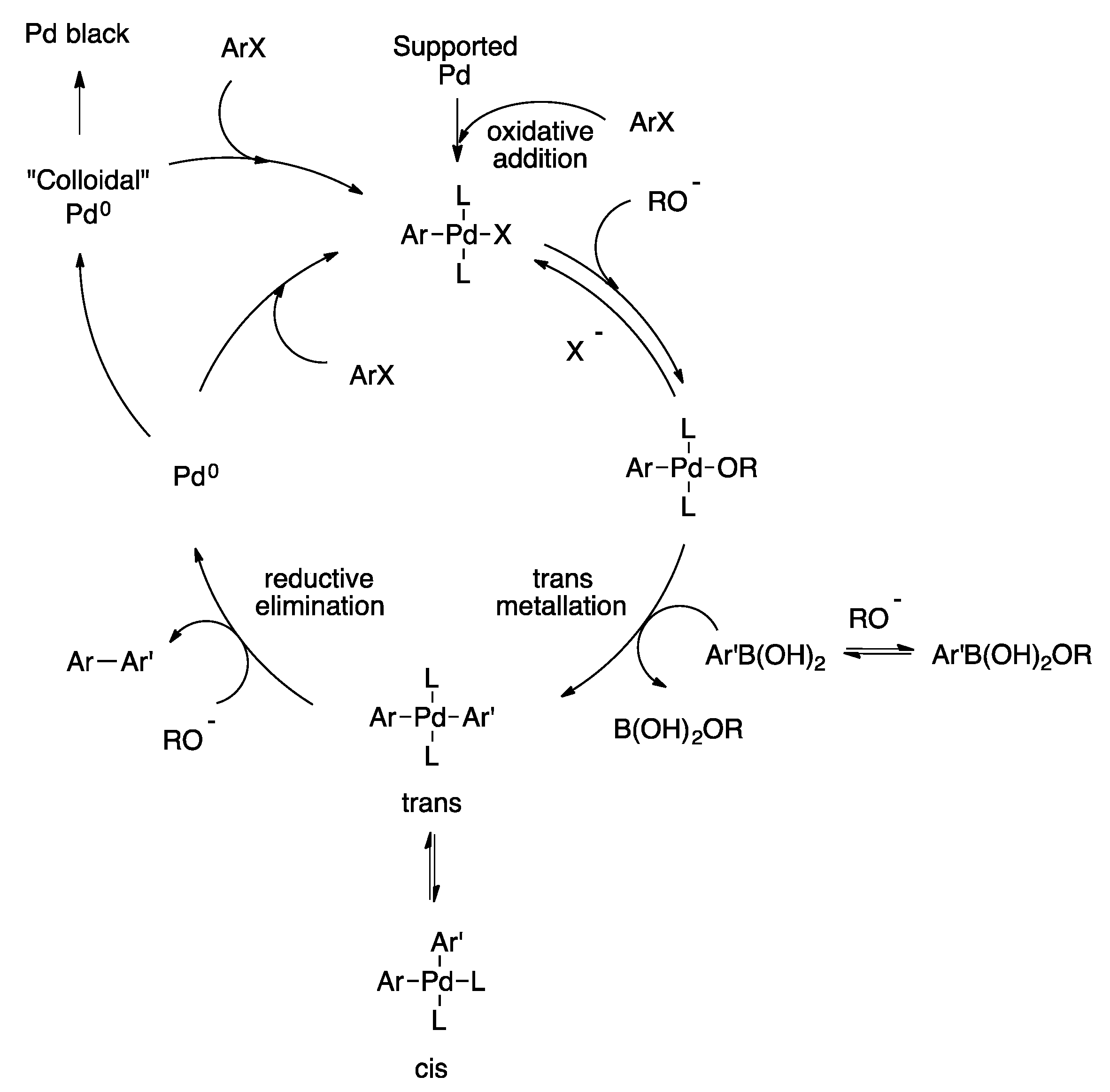
Scheme 1. Mechanism of the homogeneous Suzuki-Miyaura reaction. Scheme... | Download Scientific Diagram
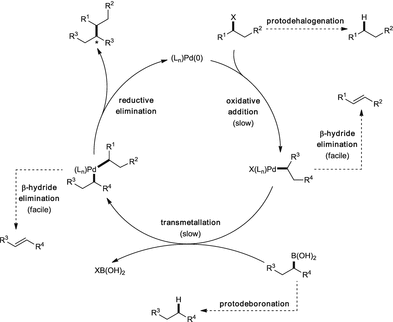
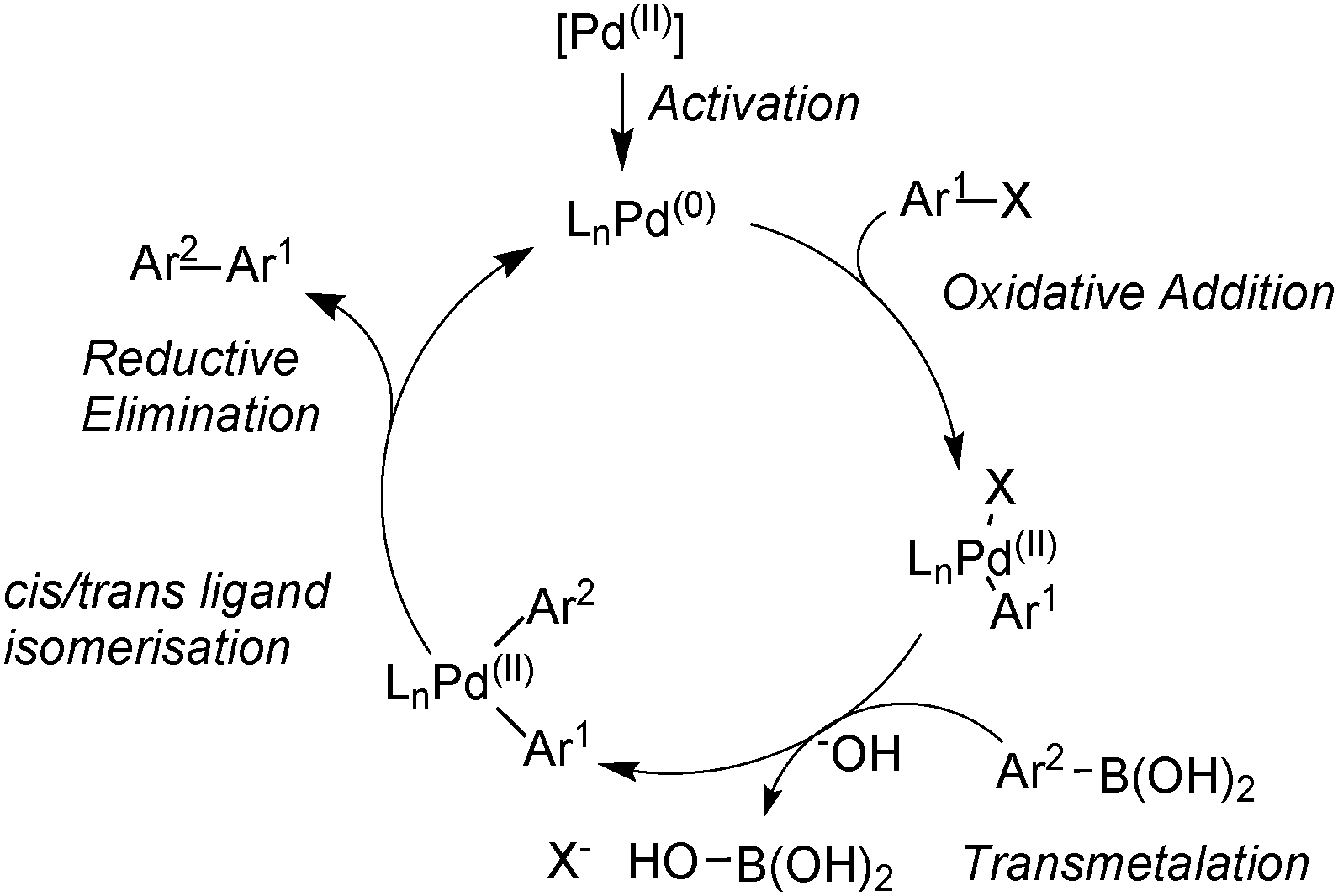
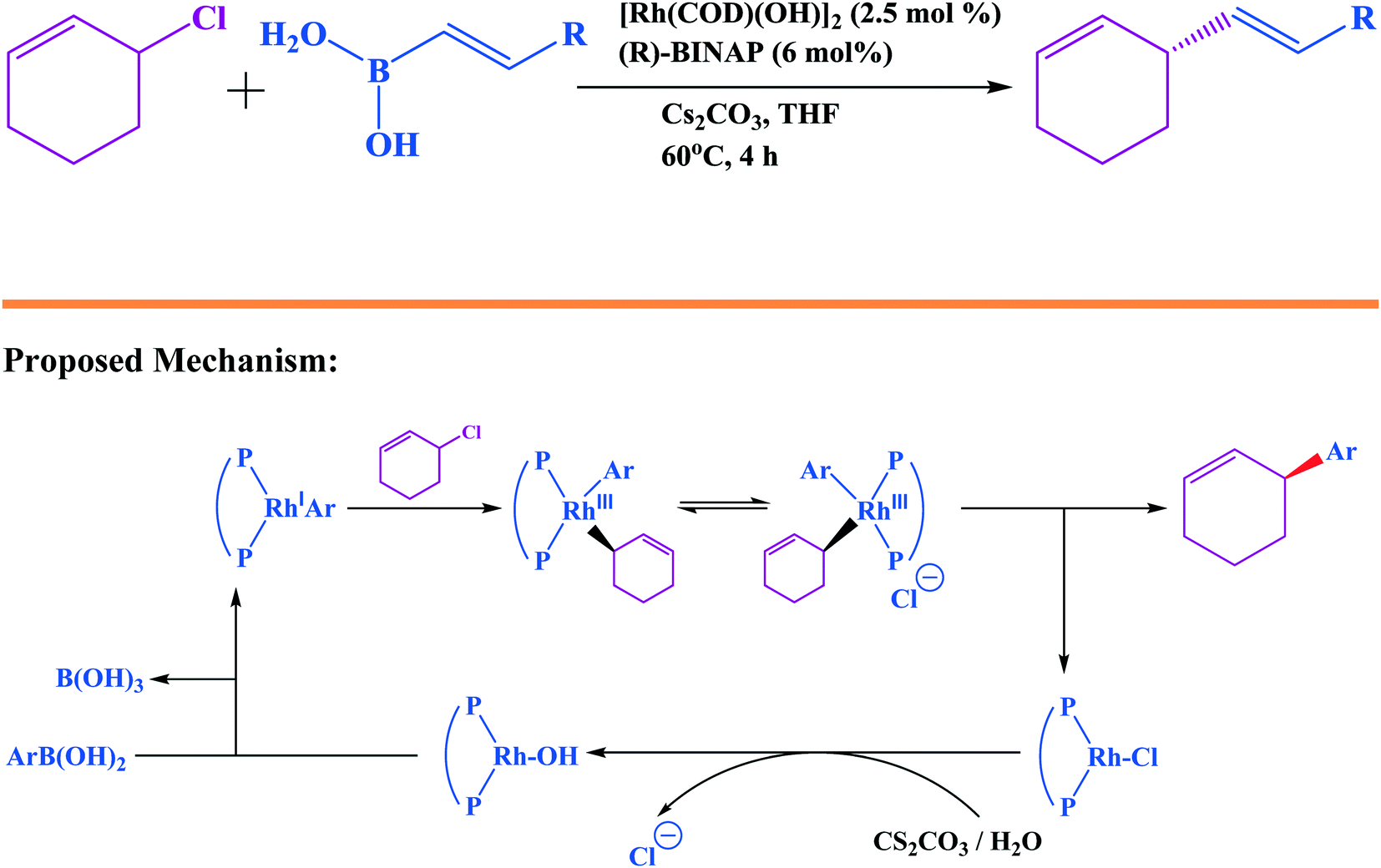
At the Forefront of the Suzuki–Miyaura Reaction: Advances in Stereoselective Cross-Couplings | SpringerLink








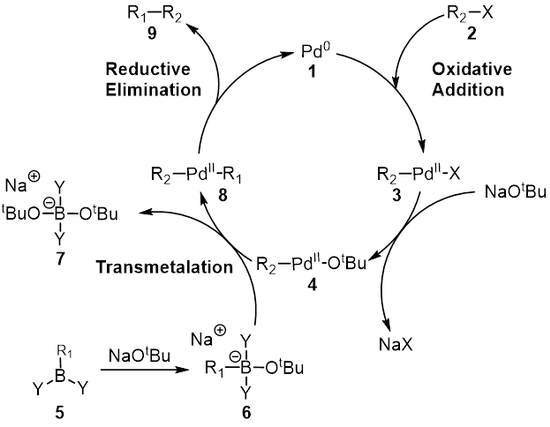
![46] Suzuki Cross Coupling 1979 – ChemInfoGraphic 46] Suzuki Cross Coupling 1979 – ChemInfoGraphic](https://cheminfographic.files.wordpress.com/2017/11/46_suzuki_coupling1.jpg)