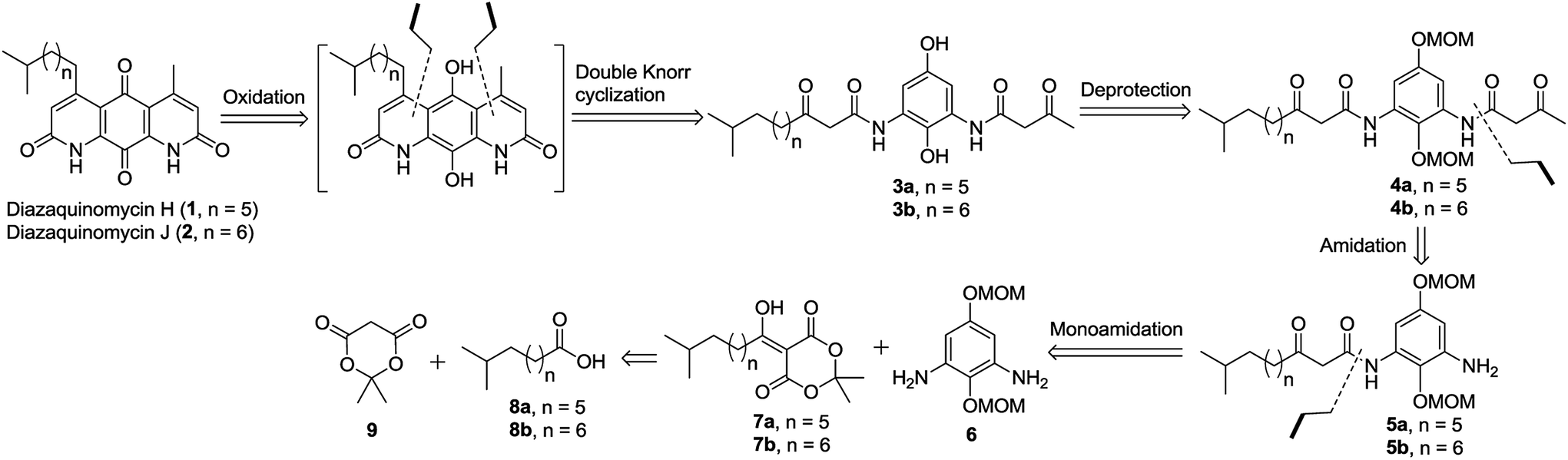
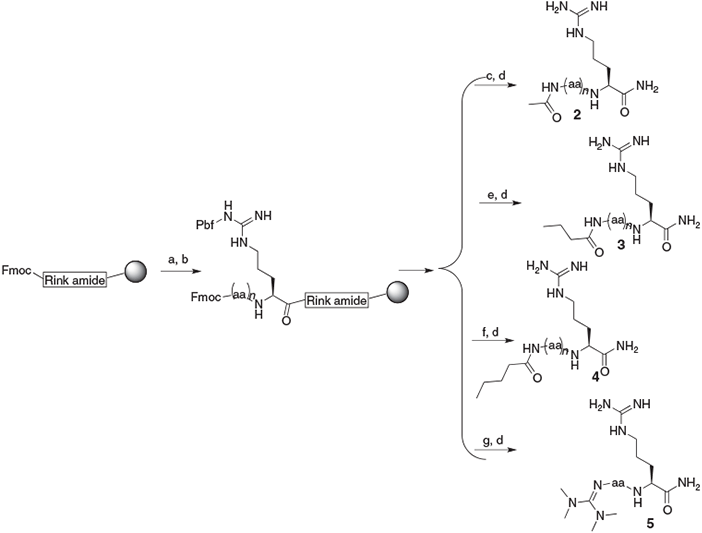
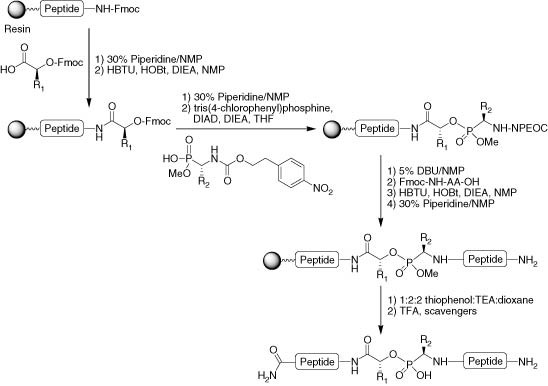
Advances in Fmoc solid‐phase peptide synthesis - Behrendt - 2016 - Journal of Peptide Science - Wiley Online Library

An important side reaction using the thiol, 3,6‐dioxa‐1,8‐octanedithiol (DODT), in 9‐fluorenylmethoxycarbonyl‐based solid phase peptide synthesis - Harris - 2014 - Journal of Peptide Science - Wiley Online Library

Solid-Phase Peptide Synthesis of Dipeptide (Histidine-β-Alanine) as a Chelating Agent by Using Trityl Chloride Resin, for Removal of Al3+, Cu2+, Hg2+ and Pb2+: Experimental and Theoretical Study

Solid-Phase Peptide Synthesis of Dipeptide (Histidine-β-Alanine) as a Chelating Agent by Using Trityl Chloride Resin, for Removal of Al3+, Cu2+, Hg2+ and Pb2+: Experimental and Theoretical Study
An important side reaction using the thiol, 3,6â•'dioxaâ•'1,8â•'octanedithiol (DODT), in 9â•'fluorenylmethox
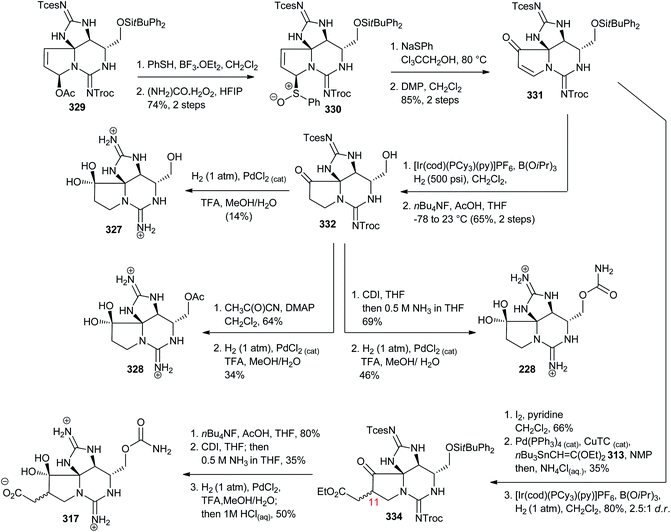
The chemistry and biology of guanidine secondary metabolites - Natural Product Reports (RSC Publishing) DOI:10.1039/D0NP00051E

1,4-Benzenedimethanethiol (1,4-BDMT) as a scavenger for greener peptide resin cleavages - RSC Advances (RSC Publishing)

WO2014033466A1 - Method and compositions for removing acid-labile protecting groups - Google Patents

The synthesis and reactivity of optically pure amino acids bearing side-chain thioamides - Journal of the Chemical Society, Perkin Transactions 1 (RSC Publishing) DOI:10.1039/B004688O